Abstract
Background Induction of fetal hemoglobin (HbF) by repressing BCL11A could reduce or eliminate sickle cell disease (SCD) clinical manifestations. The BCH-BB694 lentiviral vector (LVV) encodes a shRNA targeting BCL11A embedded in a microRNA scaffold (shmiR) allowing erythroid-specific knockdown to induce γ-globin expression and concomitantly and coordinately repress β-sickle globin expression (Brendel et al. JCI 2016). The safety and efficacy of gene therapy (GT) with BCH-BB694-transduced autologous CD34+ cells in patients with SCD is being investigated in a pilot and feasibility trial (NCT03282656), which has now completed enrollment of all 10 planned patients. Preliminary data in the first 6 patients described robust HbF induction and a favorable safety profile (Esrick et al. NEJM 2021). Additional follow-up data including all ten pilot trial patients is discussed here with longest follow-up now > 4 years.
Methods Patients with severe SCD were screened for eligibility. Autologous CD34+ cells were collected via apheresis after plerixafor mobilization (Esrick and Manis et al. Blood Adv 2018) and then transduced ex vivo with the BCH-BB694 shmiR LVV. Gene modified cells were infused into subjects who had received myeloablative busulfan conditioning. Patients were monitored for adverse events (AEs), hemoglobin (Hb), HbF fraction, percent F cells, hemolysis markers, and SCD clinical manifestations.
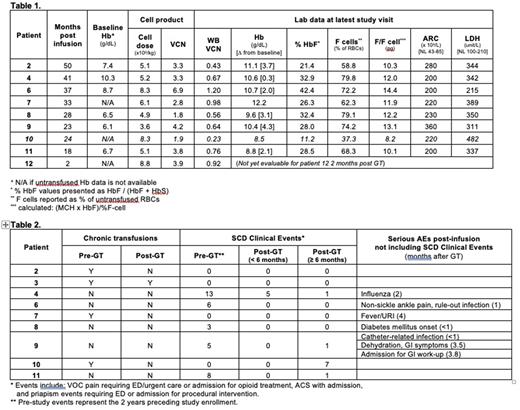
Results Ten patients (7-25 years of age at enrollment) received GT, with median follow-up of 30.5 (2-50) months. There were no Grade 3 or 4 AEs associated with mobilization, collection or infusion. Median product VCN, % transduced cells, and CD34+ cell dose in the 10 patients were 3.5 (1.8-6.9) copies/diploid genome (c/dg), 96% (62-100%), and 5.7 (3.6-8.8) x 106 CD34+ cells/kg, respectively. Engraftment of neutrophils occurred on day 22 (18-30) and of platelets on day 32 (25-62). One patient (patient 10) has low post-GT peripheral VCN, HbF, and percent F cells; most recently 0.23 c/dg, 11.2%, and 37.3% respectively. Although improved, this was considered a GT failure and the patient has been started on new SCD-directed therapies due to ongoing SCD clinical manifestations. Subsequent data described here exclude patient 10.
At the most recent study visit, the 7 untransfused patients with follow up > 6 months had mean total Hb of 10.5 (8.8-12.2) g/dL, HbF/(F+S) of 30.3% (21.4 - 42.4%), percent F cells of 70.6% (58.8-79.8%), and HbF per F cell of 12 (10.1-14.4) pg (Table 1). The lowest Hb response was seen in patient 11 who has 2-gene deletion alpha thalassemia in combination with SCD. Five patients with follow-up > 6 months (patients 4, 6, 8, 9, and 11) had frequent severe vaso-occlusive events (ACS, priapism, or pain requiring inpatient or ED opioids) as indication for eligibility, with a median of 6 (3-13) events (Table 2). From 6 months after GT through present, these 5 patients have had a median of 1 (0-1) event. Three patients with follow-up > 6 months (patients 2, 3, and 7) were on chronic transfusion regimens pre-GT. Patient 3 has continued chronic transfusions due to pre-existing moyamoya with goal of maintaining HbS at pre-GT levels. Patients 2 and 7 have received no post-GT transfusions. Patients 2, 3, and 7 have had no SCD clinical events. Mean lactate dehydrogenase and absolute reticulocyte count at last study visit were 346 (215-389) U/L and 241 (200-360) 109/L, respectively, indicating decreased but persistent mild hemolysis in all patients.
There have been no instances of graft failure, replication competent lentivirus detection, or clonal dominance in any patient. Six patients with ≥2 years post GT have had bone marrow biopsies at the 2 year timepoint without evidence of myelodysplasia. Targeted sequencing of ~95 genes frequently mutated in hematologic malignancies has been performed on peripheral blood samples every 6 months since early 2021 in patients with ≥6 months from GT. One patient had a pathogenic mutation detected in the DNMT3A gene, which was present in a stored pre-GT sample and has been present at a stable VAF <4% at 6, 9, and 12 months post-GT.
Summary Post-transcriptional gene editing of BCL11A appears safe with stable induction of broadly distributed HbF and significant mitigation of VOE events in severe SCD in all but one treated patient. Based on the results of this pilot study, a phase 2, multi-institutional trial is now open for enrollment using the same vector and approach (NCT05353647).
Disclosures
Esrick:bluebird bio: Consultancy, Other: Provides vector for trial. Heeney:Bluebird Bio: Consultancy; Oric Pharmaceuticals: Consultancy; FORMA Therapeutics: Consultancy; Novartis: Consultancy; Vertex/ Crisper Therapeutics: Consultancy. Kim:LabCorp: Consultancy; Multiple Myeloma Research Foundation: Research Funding. Kohn:Cimeio Therapeutics: Consultancy; Innoskel: Consultancy; MyoGene Bio: Consultancy, Other: Membership on Scientific Advisory Board; Pluto Immunotherapeutics: Consultancy, Other: Membership on Scientific Advisory Board; Allogene Therapeutics: Consultancy, Other: Member of Scientific Advisory Board; ImmunoVec: Consultancy; TransformaTx: Consultancy. London:Jubliant Draximage: Consultancy, Other: Data Safety Monitoring Board service; Merck: Consultancy, Other: Data Safety Monitoring Board service; ArQule: Consultancy, Other: Data Safety Monitoring Board service. Williams:Insertion Site Analysis Advisory Board, Bluebird Bio: Consultancy; Bluebird: Consultancy, Other: Provides Vector; Insertion Site Advisory Board, Biomarin: Consultancy; Scientific Advisory Board, Skyline Therapeutics: Consultancy; Chief Scientific Chair, Emerging Therapy Solutions: Consultancy; Scientific Advisory Board, Beam Therapeutics: Consultancy; Novartis: Consultancy, Other: Steering Committee (fees donated to NAPAAC); Orchard Therapeutics: Other: Provides vector; Novartis: Other: Provision of study materials, medical writing.
Author notes
Asterisk with author names denotes non-ASH members.